-
Wadiche Labs:
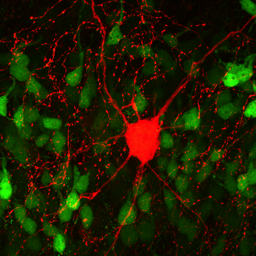
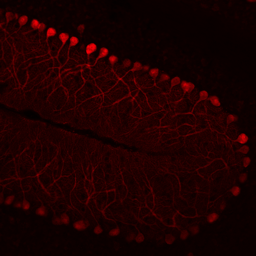
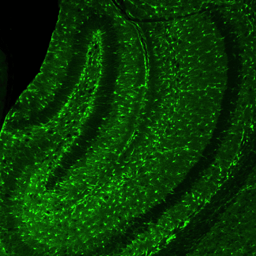
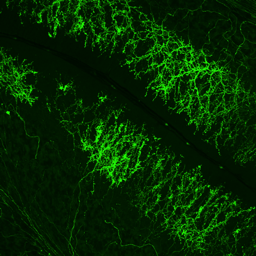
Hippocampal Dentate GyrusWe study inhibitory and excitatory neurons of the dentate gyrus, a brain region with continued neurogenesis in adulthood.
-
Wadiche Labs:
Synaptic TransmissionWe study fundamental mechanisms of synaptic transmission.
— Recent research highlights
Afferent convergence to a shared population of interneuron AMPA receptors
RL Pennock, LT Coddington, Xiaohui Yan, LS Overstreet-Wadiche, & JI Wadiche (2023). Nat. Commun. 14:3113
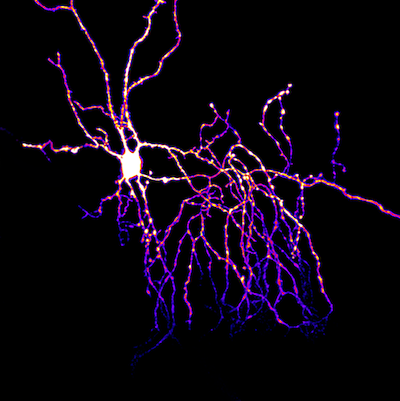
Precise alignment of pre- and postsynaptic elements optimizes the activation of glutamate receptors at excitatory synapses. Nonetheless, glutamate that diffuses out of the synaptic cleft can have actions at distant receptors, a mode of transmission called spillover. To uncover the extrasynaptic actions of glutamate, we localized AMPA receptors (AMPARs) mediating spillover transmission between climbing fibers and molecular layer interneurons in the cerebellar cortex. We found that climbing fiber spillover generates calcium transients mediated by Ca2+-permeable AMPARs at parallel fiber synapses. Spillover occludes parallel fiber synaptic currents, indicating that separate, independently regulated afferent pathways converge onto a common pool of AMPARs. Together these findings demonstrate a circuit motif wherein glutamate ‘spill-in’ from an unconnected afferent pathway co-opts synaptic receptors, allowing activation of postsynaptic AMPARs even when canonical glutamate release is suppressed.
Circadian regulation of dentate gyrus excitability mediated by G-protein signaling
JC Gonzalez, H Lee, AM Vincent, AL Hill, LK Goode, GD King, KL Gamble, JI Wadiche & L Overstreet-Wadiche (2023). Cell Reports 42:112039
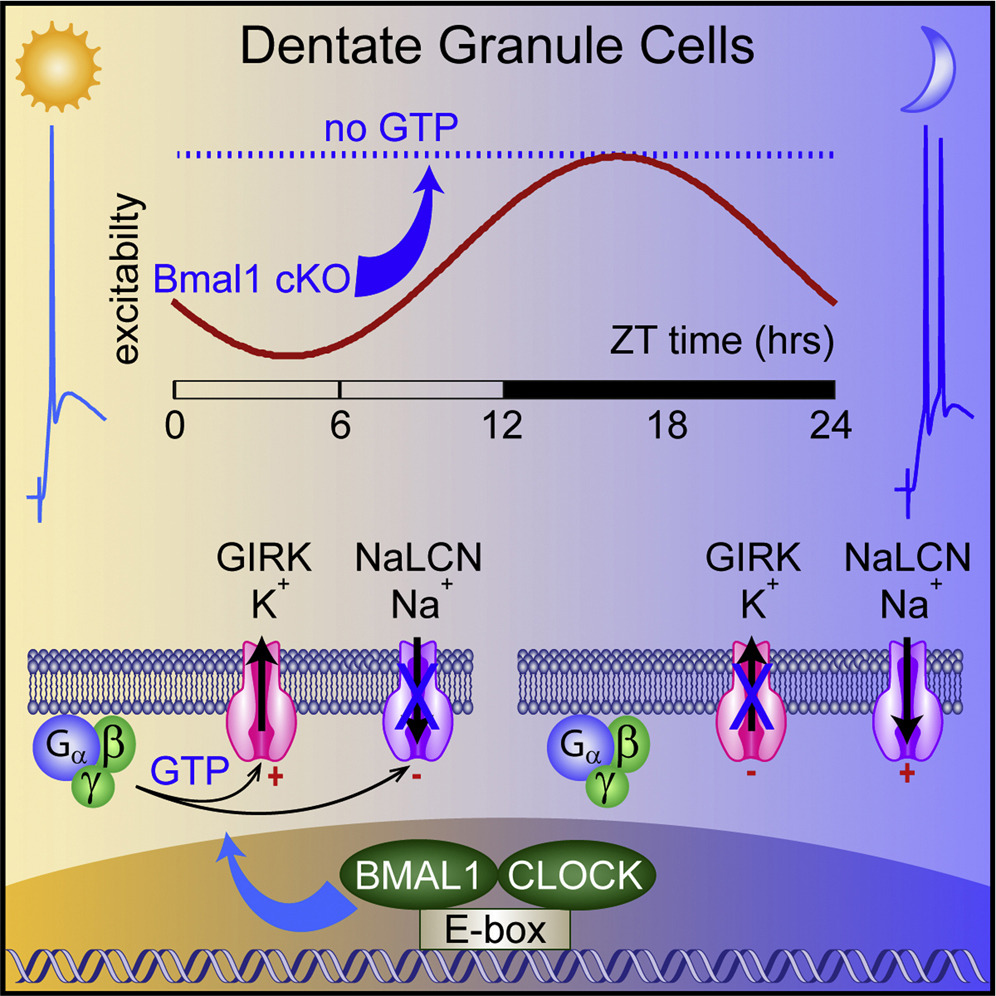
The central circadian regulator within the suprachiasmatic nucleus transmits time of day information by a diurnal spiking rhythm driven by molecular clock genes controlling membrane excitability. Most brain regions, including the hippocampus, harbor similar intrinsic circadian transcriptional machinery, but whether these molecular programs generate oscillations of membrane properties is unclear. Here, we show that intrinsic excitability of mouse dentate granule neurons exhibits a 24-h oscillation that controls spiking probability. Diurnal changes in excitability are mediated by antiphase G-protein regulation of potassium and sodium currents that reduce excitability during the Light phase. Disruption of the circadian transcriptional machinery by conditional deletion of Bmal1 enhances excitability selectively during the Light phase by removing G-protein regulation. These results reveal that circadian transcriptional machinery regulates intrinsic excitability by coordinated regulation of ion channels by G-protein signaling, highlighting a potential novel mechanism of cell-autonomous oscillations.
— Funding
Our research and discoveries would not be possible without the continuing support from the following agencies:
— Lab Heads
Linda Overstreet-Wadiche

Linda received a BS in Biology from North Park University in Chicago, IL. In 1997 she received her Ph.D. from the Department of Physiology at Northwestern University Medical School under the mentorship of Dr. N. Traverse Slater. From 1998-2004 she was a postdoctoral fellow with Dr. Gary Westbrook at the Vollum Institute, Oregon Health & Science University. Dr. Wadiche became a Research Assistant Professor at the Vollum Institute in 2004. In 2006 she joined UAB as an Assistant Professor.
Jacques Wadiche

Jacques is a graduate of Northwestern University with a B.A. in Neurobiology and Physiology. At Northwestern he gained an appreciation for basic science research and moved to Baylor College of Medicine where he worked on nicotinic receptors before enrolling in graduate school at the Vollum Institute at Oregon Health Sciences University in Portland, Oregon. He completed his postdoctoral fellowship in Craig Jahr’s laboratory at the Vollum Institute, prior to joining UAB as an Assistant Professor in 2006.